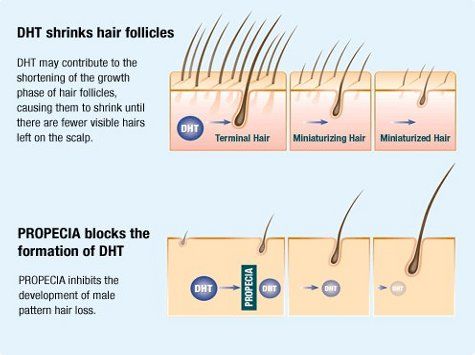
HRBR doctors often prescribe an FDA approved oral medication which blocks the conversion of testosterone to dihydrotestosterone (DHT), the form of the hormone that causes male pattern baldness. It has been available in the UK for over 25 years and widely used during this time mainly for the treatment of male pattern hair loss (androgenetic alopecia), benign prostatic hyperplasia (enlarged prostate) and several other conditions in which DHT is involved.
It is available as a 5mg and a 1mg tablet. The 1mg daily dose is generally thought to be suitable for the treatment of male pattern hair loss while for all other uses the 5mg tablet once daily is used. Due to the fact that a large number (in excess of 50) generic brands of the 5mg tablet are available, the cost of this medication has dropped dramatically in recent years and many if not most users of the medicine for hair loss choose to quarter the generic 5 mg tablet in order to achieve a financial saving of in excess of 90%. Small markets, such as the Republic of Ireland have therefore not chosen to market the 1mg branded tablet on commercial grounds. There are no reported issues related to the small dose discrepancy.
The medicine inhibits the action of the type II 5-alpha reductase enzyme that is present in and around the hair follicles of balding men with androgenetic alopecia. After two years, results showed that 83% of the men taking the medicine either kept their hair or grew more. 17% continued to lose hair while taking the medication.
The medication causes a significant drop in both scalp and blood levels of DHT. Its effectiveness is thought to be related to both of these factors. In patients taking 1-mg/day, serum DHT levels decreased by 68.4%. Serum testosterone levels actually increased by 9.1% but remained within the normal range.
Many men are concerned about reported sexual side effects with the drug. However the possible side effect of lower libido was reported in only 1.8% of the men taking the drug in clinical trials, versus 1.3% on a placebo. Further, this side effect always went away in men who stopped taking the medication, and also disappeared in most men who continued taking it.
Most reported cases of sexual dysfunction occurred soon after the medication was started. The sexual side effects were, in early trials, reported to be reversible in all men who discontinued therapy and in 58% of those who chose to continue treatment. When the medication was stopped, side effects generally went away within weeks, but occasionally took longer.
It is important to remember that when the medicine (with or without minoxidil) is discontinued, patients lose the hair that was gained or preserved by the medication, not more. In effect, you return to the level of balding where you would have been had you never used the medication(s).
Hair Loss Medication in sport
The World Anti-Doping Agency (WADA) removed the medicine from its list of prohibited medications in October 2008. It had previously been added as it was believed that it could be used to mask the use of steroids and other prohibited drugs. Better testing technology has eliminated this confusion. We would however still advise you to consult your sport’s governing body before taking the tablets.
Effects on PSA
The medication causes an approximate one third decrease in serum PSA (prostate specific antigen) in normal men (from 0.78ng/ml to 0.52 ng/ml). It may also modulate the rise of PSA levels in patients with prostate enlargement and in patients who have developed prostate cancer.
Since PSA is used as a screening test for the development of prostate cancer there is a concern that the use of the medicine may interfere with the detection of these conditions should the testing clinician not be aware that the patient is taking it. It is important, therefore, that your family doctor or urologist is aware that you are taking the medicine so that they can take into account the effect that it may have on your PSA and modulate the normal values for PSA accordingly.
It is possible that the long term use of the medicine may actually decrease the incidence of prostate disease. A major study, the Prostate Cancer Prevention Trial, documented a 25% reduction in prostate cancer in users.
Long-term benefits and risks
The effects of the medicine are confined to areas of the scalp where hair is thinning, it does not seem to stimulate hair in areas that are completely bald. Therefore, the major benefit seems to be in its ability to slow down or stop hair loss, or improve the quality of hair in parts of the scalp that are thinning. The long-term ability of the medicine to maintain one’s hair is unknown. Results generally peak around one year and then are stable in the second year or decrease very slightly.
Medical treatment and hair transplantation
The medication has been shown to be a useful addition to surgical hair restoration for a number of reasons; it works best in the younger patient who may not yet be a candidate for hair transplantation; it is less effective in the front part of the scalp – the area where surgical hair restoration can offer the greatest cosmetic improvement, it can regrow or stabilize hair loss in the crown where hair transplantation may not always be advised due to limited donor hair.
An estimated 1 million men in the United States take the medicine daily. Most men taking the medicine experienced an increase in hair count, a decrease in hair loss, and an improvement in appearance. It is important, however, to have realistic expectations as results take time. If you are not willing to commit to taking this medication for at least 12 months, it’s probably not worth starting. Secondly, it must be taken on an ongoing basis to maintain the benefit. Those who benefit from both the surgical and medical treatments are most likely in the long run to achieve the best cosmetic result. In addition, men who are already very bald are less likely to benefit from taking it because the hair follicles may not be salvageable. Men who have early thinning in the back and top have a better chance to see some benefit, but as noted above, only for as long as they continue taking the medication.
Important information about pregnancy
As outlined above, the type II 5-alpha reductase inhibitor, works by inhibiting the conversion of testosterone to dihydrotestosterone making this medication extremely useful in treating male pattern baldness. However, because of this ability to affect testosterone, exposure to the medicine could have the potential to cause abnormalities in the sexual organs of male foetuses.
For this reason, women should not take or handle the tablets when they are or may become pregnant as it is known women who genetically lack the enzyme 5 alpha-reductase responsible for the production of DHT tend to have male babies with under developed external genitalia at birth. These children, however, fully develop their genitalia when they enter their teens and start to produce their own male hormones. The medicine could, at least theoretically, produce a similar result in male babies and consequently must be avoided.
Questions have been raised as to whether women who are pregnant or trying to conceive are at any risk if their partner is taking the medicine. To date, animal studies on pregnant monkeys using intravenous doses as high as 800mg/day (which is up to 120 times the highest estimated exposure of women to the drug from the semen of men taking 5mg/day) resulted in no abnormalities at all. However, for peace of mind, to prevent even this tiny theoretical risk, men who are concerned can either stop taking the drug or use barrier contraception to prevent any possible exposure during pregnancy. For further information on the use and avoidance of medications during pregnancy see www.OTISpregnancy.org
Update on Medical treatment for hair loss
Objective overview of medications approved by the FDA in the United States (and other state medical treatment licensing agencies throughout the world) for the prevention and treatment of androgenic hair loss (male pattern hair loss).
Medications are used both in the management of early hair loss and in conjunction with hair transplantation for the treatment of the more advanced stages of this condition. Indeed, some hair surgeons would consider their use a mandatory addition to surgical hair transplantation.
It is generally felt that these treatments are more effective in the 18-41 age group, but exceptions exist and significant benefits from these medications are frequently seen outside this age group.
The most effective of these medications was originally licensed for treatment of benign enlargement of the prostate gland in January 1992 and subsequently licensed for the treatment of male pattern hair loss in December 1997. How it works in both conditions is identical, the inhibition of Type II 5-alpha reductase, an intra-cellular enzyme that converts testosterone to 5-alpha dihydrotestosterone (DHT). Both benign enlargement of the prostate and male hair loss is dependent on the presence of this hormone, which although vital in the genital development of the male foetus, does not appear to have a critical role in the adult male.
The medicine has been extensively studied. An EBSCO Discovery Service search of the literature (24th October 2016) show that 18,310 papers have been produced on the subject of this medication since its introduction, 2,287 of these being published since January 2014. These studies have extensively evaluated the efficacy and the safety of this medication and as a result of this research, the medication has been established as an important, effective and safe treatment of both male pattern hair loss and enlarged prostate as well as a number of other conditions.
Sexual Side Effects
The great majority of users do not suffer any side effects from its use. Less than 2% of males report decreased libido, semen volume and erection difficulties which disappear on stopping the medicine and usually also resolve on continuing to use it. Recently, a very small number of users report persistence of the sexual difficulties. The PCPT prostate trial which looked at the sexual dysfunction in 17,313 patients, taking the medicine over a 7 year period, found that it was associated with a small average increase in sexual dysfunction that decreased over time. The anecdotal reports of prolonged sexual difficulties, however, have been criticized for their selection and recall bias and lack of psychological evaluation of the patients and the failure to rule-out the co-existence of many other causes of these symptoms.
The total freedom of 97% of users from any side effects whatsoever may lead us to the conclusion that other factors are involved in the cause of these persistent sexual symptoms. Erectile dysfunction (ED) is the most thoroughly studied sexual dysfunction in men and the most common sexual complaint of men presenting to their health care providers. Various large-scale studies (both cross-sectional and longitudinal) have indicated that the worldwide prevalence of ED is between 10% and 20%. ED is strongly correlated with aging, with a steep incline in incidence rates, from 6.5% in men aged 20 to 39 years to 77.5% in those aged 75 years and older. ED is also associated with various co-morbidities, including psychological factors, cardiovascular diseases, diabetes mellitus, metabolic syndrome, and with smoking.
As a result of the limited, and sometimes poor quality data, on the possible side effects of the medicine, much of which was not consistent with the experience of clinicians using this medication in practice, the Boston Collaborative Drug Surveillance Program at Boston School of Public Health, the New England Research Institute and the Kingston General Hospital, Queen’s University, Ontario under took a major study of the risk of erectile dysfunction associated with the use of the medicine in the treatment of both benign prostatic enlargement and male pattern baldness. This study used the British Clinical Practice Research Database that contains data on some 10 million people. It would found that in the population with benign prostatic hyperplasia or male pattern baldness, the risk of erectile dysfunction was not increased with use of the medicine. (British Medical Journal 2016;354:i4823).
Cancer
The medicine has been investigated over a number years as a chemo-preventative for prostate cancer. Two major trails have been undertaken. The statistical results are currently difficult to interpret as some forms of prostate cancer are reduced and a smaller fraction of higher grade cancers appear to be increased in incidence. The latter, according to the statisticians, is due to the medicine reducing the prostate size and consequently increasing the likelihood of a positive biopsy hit in the reduced target gland.
Notwithstanding the difficulties involved in the interpretation of these studies and despite the lack of FDA approval for its use as a chemo-preventative, many leading prostate surgeons use the medicine in patients who are at high risk of developing prostate cancer as might be suggested by a very strong family history and also in those who have been shown to have pre-cancerous conditions of the prostate diagnosed on biopsy.
The association between the medicine and the development of breast cancer is mentioned in the side effect data sheet. No statistical data exists to substantiate an increased incidence of breast cancer in users of the medicine and the association is purely theoretical. This speculative risk has led clinicians to advise users to check for breast lumps which all males should do in any event as the total world incidence of male breast cancer is increasing possibly due to the rising levels of environmental oestrogenic chemicals. The rare, non-cancerous enlargement of the male breast (gynaecomastia) possibly associated with the medicine as well as many other prescribed and recreational drugs as well as food stuffs is not regarded as a pre-cancerous condition.
Depression
Depression has recently been observed in patients taking the medicine. The incidence of this is very low, amounting to a small fraction of the very significant total incidence of depression associated with untreated hair loss.
Other Side Effects
Other side effects are also mentioned in the side effect data sheet. These are likely to represent symptoms resulting from the rare allergy to the medication as they are commonly associated with allergic reactions to other medications, environmental chemicals and food stuffs.
To put the above in context, our usage of and regular monitoring of patients taking the medicine over many years shows that over 97% of users reported no side effects whatsoever from this medication.
A recent review (August 2016) by the SWOG Statistical Center, Fred Hutchinson Cancer Research Center, Seattle, WA, Cancer Therapy and Research Center, University of Texas Health Science Center at San Antonio, San Antonio, TX, Columbia University, New York, NY, Fred Hutchinson Cancer Research Center, Seattle, WA, Division of Cancer Prevention, U.S. National Cancer Institute, Rockville, MD, concluded that overall, there is little need to be concerned about long-term non-cancer consequences of use in those who use it for treatment of symptomatic BPH, hair loss, or prevention of cancer.
To advise patients not to use this treatment would not only put ourselves out of line with scientifically based, ethically motivated hair clinicians throughout the world but would also deny our patients an opportunity to choose an important, safe and effective treatment for their hair loss, a condition frequently associated with loss of self esteem and quality of life as well as psychological discomfort. As ethical medical practitioners, we need to follow and be guided by peer reviewed mainstream clinical and scientific studies published in major international journals rather than the internet blogs and chat rooms, many of whom have other agenda.
Topical Application
Interesting work is being undertaken by a Swiss group, International Journal of Clinical Pharmacology and Therapeutics, Vol. 52 – No. 10/2014 (842-849), on the use of a 0.25% topical solution. They published their initial results in 2014 which showed a strong and similar inhibition of plasma DHT after 1 week of treatment with the tablet and topical formulations; although the plasma exposure was significantly lower with the topical than with the tablet. No progress, to our knowledge, towards an approved FDA product has been made but several non FDA approved topical preparations are obtainable.
For some individuals there may be a concern that these formulations have not undergone the rigorous testing of a clinical trial, and are not, to date, FDA approved. However, this is not a new drug in any way, only the method of application and delivery system is different. It would be incredibly surprising if there were any new adverse effects – especially since there will be much less total exposure.
The only issue of concern is the possibility of inadvertent exposure of a pregnant woman to the topical drug. There is a warning to this on the drug labeling of these products so as to avoid this risk.
These topical preparations are not intended to replace oral tablets as a primary treatment. They were formulated for those patients that did not wish to take the medication in tablet form and wanted to effectively prevent further hair loss.
Currently, we understand, that there is a preparation available by prescription in Canada. The market place is replete with other topical preparations using both a 0.1% and 0.25% formulation.
A combined topical product is manufactured, among others, by Kumar Organic Limited, India and Aurobindo Pharma Limited, India. This has been shown to be a superior treatment when compared to minoxidil topical solution alone. (Sheikh S et al., J Clin Exp Dermatol Res 2015, 6:1).
We mention these non FDA approved treatments for information only and as pointers as to where the next generation of hair loss treatments may possibly be going. HRBR currently confines its prescription policy to FDA approved medications and formulations.
Joe O’Connor
MB, BCh, FRCS RCPS Glasg,FRCS Edin,FRCS Eng,FRCSI.
Further information on FDA approved medications for hair loss is available from the manufacturers, Merck Sharp and Dohme, or from the electronic medicines compendium.