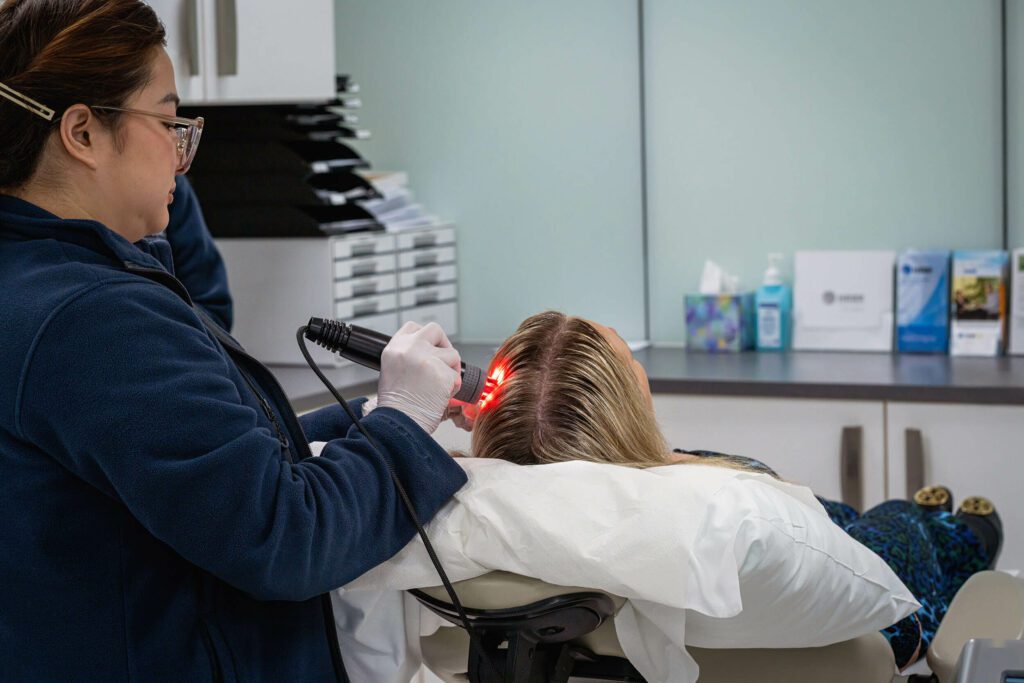
Tricopat® is a non-invasive scalp therapy designed to address hair loss and thinning in both men and women. The treatment is based in patented technology and supported by clinical research conducted in collaboration with the University of Bologna, Italy — a recognised centre for dermatological hair research.
What Is Tricopat®?
Tricopat® is a technology developed specifically for hair loss conditions such as androgenetic alopecia (male and female pattern hair loss) and telogen effluvium (diffuse shedding).1 It applies a multi-modal protocol known as TRICOGENESI®, which integrates mechanical stimulation, pressure waves, controlled micro-incisions, iontophoresis, LED photobiomodulation and the application of a serum containing preformed growth factors/peptides to improve the scalp environment and support the function of hair follicles that are miniaturised or temporarily in a resting (telogen) phase, and to encourage progression into active growth.
Tricopat® is needle-free, painless and requires no surgical intervention — making it suitable for patients seeking non-surgical alternatives or complementary therapies to established medical or surgical approaches.
How Tricopat® Works
Tricopat® engages multiple mechanisms aimed at enhancing hair follicle function and scalp physiology:
- Skin Patting & Micro-Incisions: The device creates very shallow, controlled micro-incisions (0.25 mm depth), while generating rhythmic mechanical stimulation across the scalp. This action is believed to enhance blood microcirculation, trigger natural repair responses, and prime the tissue for improved absorption of active compounds.2
- Pressure waves: Radial pressure waves are expected to increase oxygenation and circulation in the scalp, facilitating nutrient delivery to the follicular unit and further supporting cellular metabolism.3
- Iontophoresis: A mild electrical current assists in transdermal delivery into the skin of preformed growth factors/peptides and other active ingredients contained in a topically applied serum. This needle-free ‘injection-like’ delivery system enhances penetration without breaking the skin barrier significantly.4–6
- LED Photobiomodulation: Red LED light has been described in scalp/hair treatment. In this setting, it is used to stimulate metabolic activity in scalp tissues.7
Alongside the application of preformed growth factors/peptides contained in Tricopat serum, these actions aim to prolong the anagen (growth) phase of the hair cycle, improve the health of existing follicles, and support regrowth of hair shafts that may have entered prematurely the resting (telogen) or shedding phase.
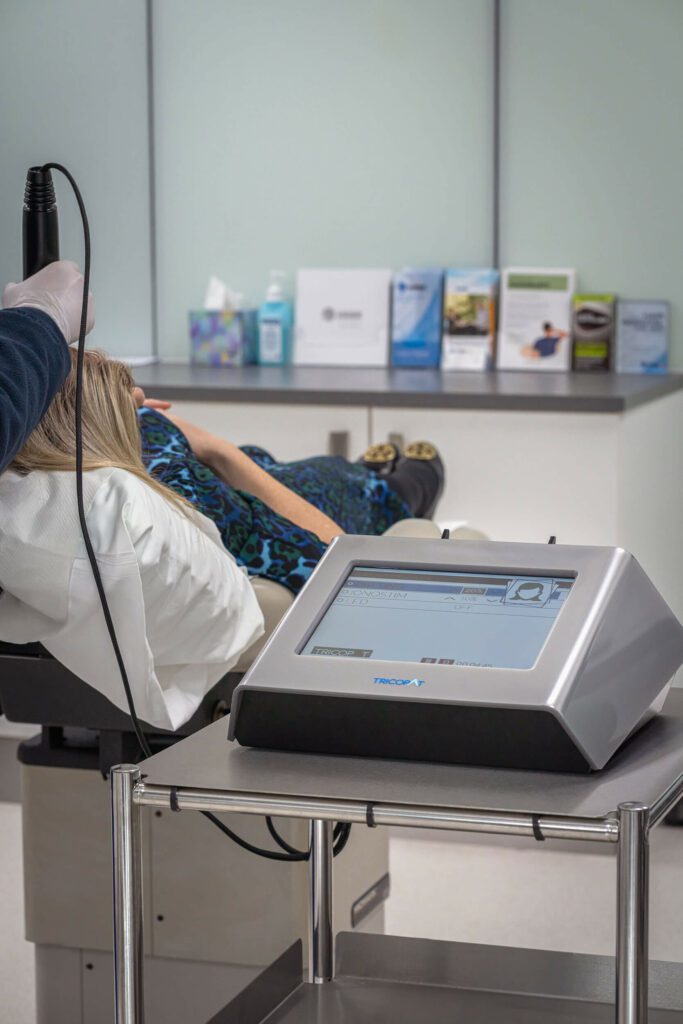
Clinical Evidence and Validation
The Tricopat protocol has been evaluated in clinical settings, most notably in studies involving both male and female patients with androgenetic alopecia and telogen effluvium, as well as selected inflammatory or diffuse alopecia presentations reported in small clinical series.1,3,8 These investigations documented measurable and meaningful increases in hair density and shaft thickness, following a course of treatment, with patients also reporting subjective improvements in hair quality.
The Treatment Experience at HRBR
At HRBR, Tricopat treatments are tailored to each individual. Most treatment plans start with a course of six sessions spaced one month apart, after which maintenance treatments will be recommended at intervals spanning 3, 6, or 12 months depending on clinical response.
A typical session lasts approximately 45 minutes and is described as comfortable by most patients. There is no downtime, and patients can resume normal activities, including returning to work or washing their hair the same day.
Unlike procedures such as PRP (platelet-rich plasma), Tricopat does not involve blood draws, injections, or the associated risk of bruising and swelling.
Who May Benefit from Tricopat®
Tricopat® is indicated for individuals experiencing:
- Telogen Effluvium, where diffuse shedding occurs due to stressors such as illness, surgery, medications, weight loss or hormonal shifts.
- Male and Female Pattern Hair Loss, where genetic and hormonal factors lead to progressive thinning of hair.
Costs
The cost per Tricopat treatment at HRBR is €300. In addition, a reusable treatment head costing €100 is required; this head is reused for a patient’s own treatments and generally lasts for approximately six sessions.
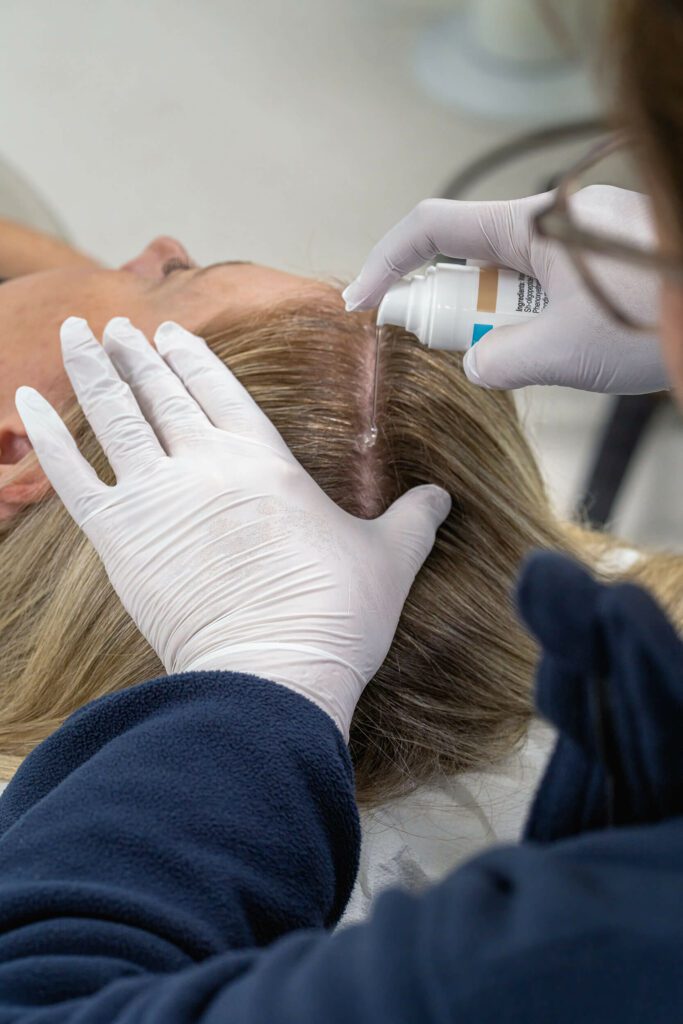
Safety and Suitability
Tricopat® is generally well-tolerated. Because it is non-invasive and does not breach the dermal layer deeply, the risk profile is low. Some patients may experience mild scalp sensitivity, but significant side effects are uncommon.
Patients with certain medical conditions — such as those with implanted electrical devices (e.g., pacemakers), pregnant patients, or those with metal cranial implants — may not be suitable candidates and should discuss alternatives with their clinician.
Note that it is not possible to treat patients with hair extensions.
Concluding Perspective
Tricopat® offers a research-tested, non-surgical option for individuals seeking an alternative or complementary approach to traditional hair loss treatments. By integrating mechanical stimulation, growth factor delivery, and advanced photobiomodulation, the protocol aims to enhance scalp health and support hair growth in a comfortable and repeatable therapy.
Next Steps
If you would like to learn more about Tricopat or discuss whether it may be appropriate for you, please contact HRBR to discuss your options and arrange a consultation.
References:
1 Alessandrini AM, Bruni F, Piraccini BM, Starace M. The Effectiveness and Tolerability of Preformed Growth Factors Vehiculated Through Iontophoresis on Patients with Androgenetic Alopecia and Telogen Effluvium: A Clinical Study. Dermatol Pract Concept 2021; 159:2021082.
2 Vettorato E, Volonté P, Musazzi UM, et al. Skin microincision technique to enhance drug penetration for the treatment of keloid and hypertrophic scars. Int J Pharm 2025; 671. doi:10.1016/j.ijpharm.2025.125259.
3 Cedirian S, Pampaloni F, Quadrelli F, et al. Efficacy of Skin Patting and Iontophoresis with Dutasteride Gel in Male and Menopausal Female Androgenetic Alopecia: A Pilot Study. Dermatol Ther (Heidelb) 2025; 15:3419–24.
4 Hama S, Kimura Y, Mikami A, et al. Electric stimulus opens intercellular spaces in skin. J Biol Chem 2014; 289:2450–6.
5 Hasan M, Khatun A, Kogure K. Iontophoresis of Biological Macromolecular Drugs. Pharmaceutics 2022; 14. doi:10.3390/pharmaceutics14030525.
6 Kajimoto K, Yamamoto M, Watanabe M, et al. Noninvasive and persistent transfollicular drug delivery system using a combination of liposomes and iontophoresis. Int J Pharm 2011; 403:57–65.
7 Umino Y, Denda M. Effect of red light on epidermal proliferation and mitochondrial activity. Ski Res Technol 2023; 29:1–6.
8 Starace M, Cedirian S, Quadrelli F, Piraccini BM. Iontophoresis as a potential treatment for alopecia areata incognita. Ital J Dermatology Venereol 2024; 159:1–2.